
LAB # 3 - RELATIVE AND "ABSOLUTE" GEOLOGICAL TIME

LAB # 3 - RELATIVE AND "ABSOLUTE" GEOLOGICAL TIME
In this lab we will look at: 1) relative stratigraphy, which uses the principles of stratigraphy discussed in class to determine relative age; 2) biostratigraphy, which uses fossils to assess relative age; and 3) radiometric dating, which uses the decay of various isotopes to put a numeric age on the rock of interest. There are other methods of dating, such as magnetostratigraphy and correlation by event horizons, and these will be discussed in class. Write your answers in your laboratory notebooks.
| m.y. | million years. e.g. "the Cretaceous period lasted 80 m.y." |
| Ma | age in millions of years. e.g., "the Cretaceous period lasted from 145 to 65 Ma" |
When subdividing the time periods you may see the terms Lower, Middle,
Upper, or Early, Middle, Late.
Geologists use Lower & Upper to refer to rocks, e.g. "this tyrannosaur comes from Upper Cretaceous strata"
Early and Late are used to refer to time, e.g. "this tyrannosaur lived in the Late Cretaceous".
The fundamentals of relative stratigraphy were first outlined by Steno in the 17th century. Steno's principles are as follows:
1. Principle of superposition of strataThese principles allow for the relative order of events in time to be worked out from the geometry of strata. Using these principles, work out the following two problems.
2. Principle of initial horizontality of strata
3. Principle of stratal continuity
4. Principle of cross cutting relationships
Problem 1
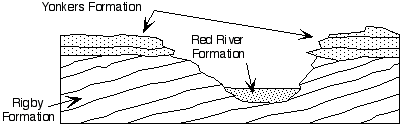
The diagram above shows a cross section through a canyon and the rocks
surrounding it. All of the units are sedimentary.
1. What is the order in which the rock and sediment units formed?
2. What principles have you used to deduce this order? Explain your choice.
Problem 2
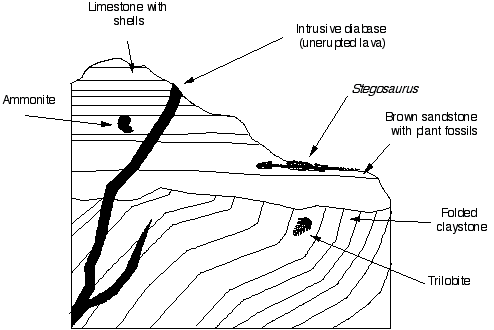
The diagram above shows a more complicated cross section through a hillside and the rocks in it.
Ammonites: marine Mesozoic animals
Stegosaurus: Late Jurassic dinosaur
2. What principles have you used to deduce this order? Explain your choice.
3. What age/range of ages (in periods, not eras) are the different layers of strata?
4. What environments were the units formed in?
PART 2. BIOSTRATIGRAPHY AND SERIATION
Biostratigraphy is the method of using fossils to determine the age of one stratigraphic section relative to another.
It deals with the inference of relative time relationships based on the distribution of organisms, or more meaningfully, those groups of organisms called taxa. Although biostratigraphy can operate at coarse taxonomic levels (e.g. Ornithischia = Mesozoic), this level of resolution is usually too coarse to be of real use. The traditional methods of biostratigraphic correlation are subjective and sometimes non-repeatable. Traditionally, a stratigraphic section is measured and a taxon-by-level matrix is produced. Simply, the occurrence of various taxa are plotted relative to their stratigraphic level (up and down section), and we then "correlate by eye".
In order to remove some of the subjectivity of this method of fitting by eye, some semi-quantitative and hence explicit and repeatable methods have been developed. These methods are also much easier to teach than the mushy "eye ball" method.
In general, when correlating two sections, we want to 1) maximize the shared occurrence of taxa; and 2) minimize unsampled range extensions for particular taxa. The second is most important because these ad hoc range extensions are introduced by the theory, rather than the original data set. By "range of taxon" we are referring to the extent of its biostratigraphic occurrence, i.e. when/where it first appeared and when/where it last appeared.
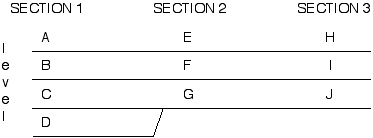
A method of biostratigraphic correlation which meets these criteria is seriation. This method was originally developed by archeologists who were trying to put different geo-graphically-isolated artifact assemblages into some kind of time order. The first step is to create taxon-by-level matrices of the various sections that have been measured (see below). Specifically, an X is placed at the level where we find a taxon. In the example given we have 3 different sections or localities, with 4 (A-D), 3 (E-G), and 3 (H-J) strati-graphic levels (i.e. layers of rock yielding fossils which we wish to correlate) respectiv-ely. 4 taxa have been identified from the various stratigraphic levels and plotted:
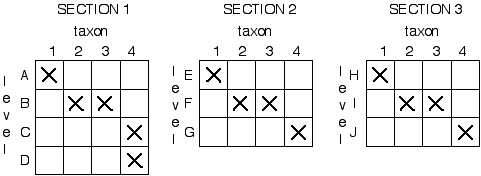
The sections are then stacked on each other :
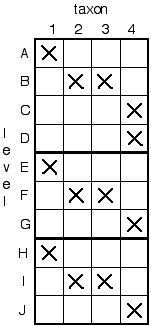
At this point, two different methods of seriation can be considered; we will use one called constrained seriation. "Constrained" means that the stratigraphic order or succession of the levels in each section we are trying to correlate can not be changed relative to each other (i.e. in section 1, layer A must always be above layer B, which must be above layer C etc.). This is important to remember when we start rearranging the layers. This basically conserves Steno's principle of superposition.
Next, the "stacked" record is rearranged so that the taxon presence data (X's) arrange themselves diagonally. In doing so, we minimize the column depth (the ad hoc range extension of the taxon), row length, and holes in the diagonal. It should become apparent that in using this method there are objective criteria for choosing one hypothesis of correlation over another. Specifically, we can count up the number of "holes" in the diagonal, and prefer the correlation which minimizes these.
After rearranging, our "stacked" record of the three sections will look like this:

In most cases, things will be much more complex because we will have to shuffle the order of the taxa as well to get the diagonal. A good rule of thumb is to position the taxa which seem to occur near the TOP parts of the section to the LEFT of the matrix, and those that seem to occur near the BOTTOM of the sections to the RIGHT. As many intermediate matrices as necessary to create the diagonal can be performed to check for "best fits" and oftentimes yield equally good results which means that there are two possible scenarios.
A correlation is made by recognizing those layers which contain the same taxa as equivalent in time. For example, we see that layers A, E, and H are stratigraphically equivalent (have taxon 1 in them);the same can be said for B, F and I (taxon 2 and 3); and C, D, G, and J. Our final correlation would therefore be:

PART 3. PRINCIPLES OF RADIOMETRIC DATING
Radiometric dating is the only method for putting an actual numerical age ("absolute age") to rocks.
Introduction
Atoms are built of protons, neutrons, and electrons. Protons and neutrons reside within the nucleus, while electrons circle the nucleus in an orbiting cloud. The majority of the mass is contained within the nucleus as protons and neutrons. Protons carry a positive charge of +1, neutrons as the name decries are neutral, and electrons carry a negative charge of -1. In most cases the number of electrons balances the number of protons in the nucleus, yielding an atom of neutral charge. If any of the nuclear particles are unstable they may decay, and thus give off energy or a particle in order to become stable. The property of giving off energy or particles is known as radioactivity. In general, radioactive decay results in the transmutation of one element, the parent, into another, the daughter. Many elements have special forms or isotopes (see definitions below) which are radioactive. As the parent "population" decreases or decays, the daughter population increases.
| Isotopes: | A class of atoms with the same number of protons but different number
of neutrons are termed isotopes of that element. e.g.:
|
||||||||||||||||||||||||||||||||||||
| Radionuclides: | The general term grouping the different classes of radioactive elements and isotopes. | ||||||||||||||||||||||||||||||||||||
| Half-life (t1/2): | The amount of time required for half of the original or parent population to decay. | ||||||||||||||||||||||||||||||||||||
| l (lambda): | Expressed as a simple probability; the probability that a radioactive nucleus will decay is expressed as the fraction of the nuclei which will decay in a unit time (usually in one year). This probability constant is known as the decay rate lambda (l). Conversely, the average life time of a radioactive atom is 1. |
Beta Radiation
For a given radioactive element, the probability that an atom will decay is a constant, just as the probability that a flipped coin will land "heads" is a constant 50%. In radioactive decay this probability is expressed as the number of decays per unit time; in coin tosses the probability is expressed as the average number of tosses giving "heads" (or "tails") per total number of tosses. However, even though the probability that an atom of an element will decay is a constant, it is quite impossible to predict when or if a single atom will decay. This is exactly the same as the realization that even though the probability of getting heads is 50%, we cannot predict how many successive "heads" will come up or whether a given toss will be "heads" or "tails". Probability is a property assessed in populations, not individuals.
While the probability that an atom of a given element will decay is a constant, the total number of atoms that will decay in a given amount of an element is obviously dependent on the number of atoms making up that amount of the element. Thus, the total rate (total decays per unit time) of an element is dependent on the amount of the element. In fact, the total rate of decay is proportional to the amount of the parent element present at a given time, and that is inversely proportional to time elapsed.
For example, if we start with 10,000 atoms of parent element "P" with a probability that any given atom will decay at a rate of 1/60 decays/min. to daughter element "D", in the first minute, something like 1/60 * 10,000 = 167 atoms of "P" will have decayed to "D". Thus, about 9833 atoms of "P" will be left. In the next minute about 1/60 * (10,000 - 167) = 163 atoms of "P" will decay, and in the next minute about 1/60 * 9669 = 161, and so on. Note that as each minute passes, there are fewer and fewer atoms of "P" left and, therefore fewer total decays per unit time.
| Minutes | # parent atoms present at start of minute | # daughter atoms present at end of minute | total decays/minute |
| 1 | 10,000 | 167 | 167 |
| 2 | 9.833 | 331 | 164 |
| 3 | 9.669 | 492 | 161 |
| 4 | 9.508 | 651 | 158 |
| 5 | 9.349 | 807 | 155 |
| 10 | 8,595 | 1,548 | 143 |
| 20 | 7,266 | 2,855 | 121 |
| 60 | 3,710 | 6,352 | 62 |
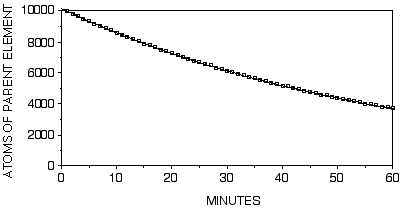
It turns out (using regression analysis) that the equation for this line has the form:
P = 10,000 * e(-0.0167 * t)
where 10,000 is the starting number of parent atoms, P is the remaining number of parent atoms, t is the amount of elapsed time since the process began, and 0.0167 is the decay rate which is this case is 1/60.
If we were to carry this process out for 400 minutes the result would look like this:
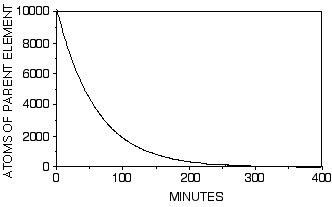
The equation of the line would still be as it was for just 60 minutes. Note that the number of parent atoms gets very small but never quite reaches 0.
The shape of this curve describes what is called an exponential relationship. Another way of saying this is that the number of atoms of the parent element is decaying geometrically. It turns out that for any case of radioactive decay, the decay equation is always:
Pf = P0 * e(- l * t)
where:
P0 is the starting number of parent atoms
Pf is the remaining number of parent atoms
t is the amount of elapsed time since the process began
l (lambda) is the decay rate
This is called the fundamental age equation.
The way we did it here is not really the correct way to do it, however.
It can be properly derived using elementary calculus, and I have described
this on page 2:15 for those who are interested.
Example 1:
The following are known:
l = 4.95 x 10-11 (1/year)
t = 2.25 x 108 years
we want to find P0 (the original P).
Pf = P0 e ( - lt )
![]()
![]()
e(- lt)
=
0.989 ![]()
As you can see, of the original 42.5 million
atoms 225 million years ago, only 0.5 million atoms (about 1.1 %) have
decayed. This is why having a large sample containing radioactive atoms
is important when we want to measure over a very short period of time (hours,
days).
Half Life
A convenient way to compare the amount of time it takes for an atom to decay is to look at the amount of time it takes for half of the original sample to decay, i.e. their respective half-lives. It is important to remember that atoms have no memory. Thus if we think of time in terms of half lives, at one half life half of the original sample has decayed, at two half lives one quarter of the original sample remains, at three half lives one eighth of the original sample remains, etc. To calculate the half life we use the following equation (for derivation see p. 2:17):
half life = t1/2 = 0.6931 / l
Example 2:
Assumptions using radiometric dating
The basic assumptions of radioactive dating are what we went over in class:
1. When a crystal of a mineral forms it starts
with some amount of a radioactive element.
2. As the "parent" radioactive element decays,
the "daughter" element stays locked within the crystal.
3. No "extra" of the daughter-type element can
be present.
4. The decay rates of the element must be known.
5. Then the ratio of daughter to parent can be
used to calculate the age of the formation of the crystal.
If these assumptions are true, we can date the
formation of a mineral within the limits of precision set by our measuring
instruments if we solve for t.
Simple Inverse Growth Equation
Unfortunately we usually do not know how much of the original parent there was at time (t)= zero (P0) because some of it has decayed to create daughters, and no-one was there to measure at t = 0. Again using some algebra we can derive an equation which expresses t as a function of the daughter parent ratio (D/P) (see p. 2:18 for derivation). This equation is:
![]()
This is known as the simple inverse growth equation.
In addition,
D = P0 - Pf
| where | D = # of daughter atoms produced by decay of the radioactive parent |
| P0 = initial number of parent atoms | |
| Pf = final number of parent atoms |
Example 3:
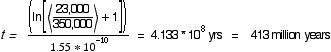
Unfortunately it is very difficult and/or
very expensive to directly measure the number of atoms of a certain element
(and its isotopes) in a sample. Instead what is normally done is to express
the parent in terms of a percentage. This is usually the case in Potassium-40
(40K) dating where the amount of Argon-40 (40Ar)
produced is usually very small. So, we have to experimentally determine
the amount of both 40K and the volume of radiogenic
40Ar,
not the actual number of atoms.
2. Measure the volume of 40Ar.
3. To obtain the 40Ar/40K ratio we use the following formula:
40Ar/40K = 1463 x [40Ar (volume) /K%]
4. To now use this, we multiply the "corrected" Ar 40/40K ratio of atoms by the fraction of decays of 40K that actually become Ar 40 which is given by the equation:
l / le
![]()
Problems
Here's what you have been waiting for, nifty problems
that take almost no time whatsoever to complete! Just "plug and chug" with
the correct equations. If your calculator does not have an exponential
key ("e to the x") you can also use [inv][lnx] to do the same thing.
| Question 2: | In the above example, what type of radioactive decay does 40K undergo? |
| Question 3: | A mineral has crystallized in an extrusive eruption
(lava flow) that scared the dinosaurs right before they went extinct at
the Cretaceous/Tertiary boundary 65 million years ago. If this particular
crystal sample started with 1 billion atoms of Rubidium-87 (87Rb),
how many 87Rb atoms are remaining today?
Also, if 87Rb decays into Strontium-87
(87Sr) how many daughter atoms would you
expect to find in the mineral today assuming that there weren't any
87Sr
atoms in the crystal when it cooled?
The decay constant lambda (l ) for 87Rb is 1.42 x 10-11 (1/yr). |
| Question 4: | In the above problem you have been given l of 87Rb, what is its half life in years? in millions of years (my)? Construct a graph showing the decay of 87Rb assuming an initial concentration of 1. How much of the original 87Rb is left after 2.93 x 1011 years (hint: you can express this as a fraction) and how many half lives is this? |
| Question 5: | The Palisades Sill is an igneous intrusion that
created the cliffs along the southern portion of the Hudson River. Biotite
(a type of the mineral mica) was extracted from a sample taken from one
of the exposed areas. Careful extraction yielded a 40Ar/40K
ratio of 0.0119. A friend of yours was kind enough to tell you that the
total decay constant (l
) for 40K is 5.81
x 10-11 (1/yr) and that l
/ le
=
0.9674
What is the age of the sill, and can you tell me if there were dinosaurs roaming around at this time? |
The measurement of radioactive decay is only accurate when the number of atoms of the radionuclide is large, since many of the contains are incredibly small. Thus, you need enough atoms in front of you (in the sample) to be able to measure the small percentage of the ones that actually decay in a relatively small time period (hours, days, and/or months). The rate of change in the number of atoms is:
![]()
where dP = the change in the number of atoms and dt = the change in time. The number of decays that are measured in a given amount of time depends upon:
1. the number of atoms you started with (P0)
2. how fast they decay (i.e. l ).
For the given time interval dt, the change in the original population of atoms (P0) can be expressed as:
![]()
To use this equation we need either the total number of daughter atoms which form in a certain interval of time, or we need to know the number of years between the closure of the system and our observations. In order to express the loss (decay) of parent atoms as a function of time we arrange to solve for time:
![]()
Integrating both sides:
![]()
l t + c = - lnP
The constant c could be a problem; however we can get rid of it by noting that t=0:
l (0) + c = - ln P0
which simplifies to:
- ln P0 = c
If we substitute -ln P0 for c, then we get:
- lnP = lt - ln P0
rearranging yields:
lnP - ln P0 = - l t
taking the log of both sides and knowing that ln(P/ P0) = ln P - ln P0 we get:
![]()
which finally yields:
![]()
This is the fundamental age equation, where P is the number of atoms
of a given radioactive element at time t, and at time t+0, the original
number of atoms is P0.
Derivation of the Half Life Equation
To calculate the half life we set P now equal to half of the original sample, which yields:
0.5 P0 = P0e - lt
Simple algebraic rearrangement yields :
0.5 = e - lt
and taking the natural log of both sides gives us:
ln (0.5) = ln (e - lt )
which becomes:
- 0.6931 = - lt
this simplifies by dividing through by -1/ l such that:
t1/2 = 0.6931 / l
Derivation of the Simple Inverse Growth Equation
It is possible to measure the number of atoms remaining (Pf) and the number of daughter atoms that have been produces (D). If at time =0 there were no daughter type atoms in the sample, i.e. D0=0, and the system has remained closed since the crystal was formed (i.e. no gain or loss of parents or daughters except for radioactive decay) then we can determine the age of the sample.
Given that ![]() and that D = P0 - Pf (remembering that D0
= 0) then:
and that D = P0 - Pf (remembering that D0
= 0) then:
![]()
![]()
then dividing through by P we get:
![]()
in order to solve for time (t) we add 1 (one) to each side and take the natural log of each side:
![]()
![]()
![]()
Thus we get the simple inverse growth equationwhere lambda is the decay rate of the parent.