 |
II. The mass of new matter again collapsed into a disk shape
mass of dust and gas (a). The center became superheated and formed
a new star, our sun (b). From this disk of matter the planets began
to condense (c), according to the widely supported nebular hypothesis
of Immanuel Kant and Pierre-Simon
Laplace. The two strongest points in favor of this idea are: 1) that
the disk began by rotating in one direction and the rotation of all of
the planets around the sun follows the original disk; and 2) that because
the disk flattened out as time progresses, all of the orbits of the planets
(except Pluto) lie more or less in the same plane (d). Pluto is possibly
a captured giant asteroid.
III. The earth condensed in four basic steps. 1) It began to accrete from the nebular cloud as particles smashed into each other forming so-called planetesimals. These in turn collided with each other and as their mass grew began to gather material from the nebular disk. 2) As the mass of the Earth grew so did it's gravitational force and the Earth began to compress itself into a smaller and denser body. This happened about 4.5 billion years ago. 3) In the third step the compression itself began to heat the interior of the Earth; also there was heat generated by radioactive decay. The interior of the earth began to melt. Because iron is the heaviest of the common elements that make up the Earth, as the Earth began to melt droplets of melted iron began to sink towards the center of the earth, where they condensed. 4) Proceeding slowly at first it sped up to catastrophic proportions - hence it is called the iron catastrophe. Note that 3 and 4 in the figures to the right are cross sections. |
 |
At some point however, the Moon formed. Exactly how that happened is a major subject of debate. One theory claims that the Moon is a tiny planet captured by the Earth's gravity. The other is that the moon was literally splashed out of the Earth by the impact of a Mars-sized planet. The latter theory is favored now because it explains some odd but important features of the Earth's and Moon's chemical composition.IV. However, the crust finally solidified by about 3.7 billion years ago. Gasses pouring out of volcanoes and fissures, along with lava, began to accumulate, perhaps added to by the impact of a few giant comets (which are mostly gas).The Earth was reborn during the iron catastrophe and maybe again by the formation of the Moon. Any trace of surface structure was wiped out by the melting.
The gases that accumulated were those we still find coming out of volcanoes:This atmosphere would be quickly fatal to us.Water vapor (H2O)These gases combined to form:
Hydrogen chloride (HCl)
Carbon Monoxide (CO)
Carbon Dioxide (CO2)
Nitrogen (N2)Methane (CH4)
Ammonia (NH4)
Hydrogen Cyanide (HCN)
V. As the crust cooled water would condense and accumulate as oceans. This happened very soon after the crust solidified.
VI. LIFE EVOLVES
The origin of life is shrouded in mystery. But there have been significant steps towards some understanding.
Making many complex organic molecules is the first step, but apparently not a difficult one.
This was shown in the famous Miller-Urey experiments done in the 1950's.The two essential elements of Life are that the system needs to be able to replicate and it needs to be separated from its surroundings.Stanley Miller and Harold Urey were a graduate student/professor team here at Columbia. They planned to set up an experiment to see how complex organic molecules could be produced. Basically they mixed together gasses that they thought the primitive Earth would have in a jar and zapped the gases with an electrical spark. Voila! Complex organic goo collected at the bottom of the jar. Included were amino acids, the building blocks of proteins. But no life.
Such experiments have been repeated many times and it is clear that it is easy to make many complex organic compounds but none of these simple experiments produced even the basics of life.
What is still pretty much a mystery is the origin of heredity and reproduction. It's possible that the surface of clay or some other mineral acted as a template for organization, but this is very hypothetical, and key experiments have yet to be performed or even rationalized.Here is a link to many sources on new experimental work on the origin of life.
Simple self-replicating molecules developed in this so-called primordial soup of organic matter in water.B. However it happened, we are fairly sure it happened early in Earth's history because fossil carbon in 3.7 billion year old rocks and stromatolites from about the same time have the tell-tale signs of life.But once there was replication, there would be occasional errors and hence, there could be evolution.
By 3.5 billion years ago there was life, certainly with the full compliment of the basic genetic system - with DNA and RNA. (Click here if you want a basic outline of DNA and RNA)
These were cells encapsulated in membranes so their internal workings were isolated from their surroundings.
We are positive that the earliest life forms were prokaryotes,
such as bacteria and Cyanobacteria.
Evidently, photosynthesis must have started nearly at the beginning. At once that changed the world because of the release of free oxygen.
Evidence for this are stromatolites and silicified microorganisms.At first all sediments were gray, indicating reduced iron. Great gold deposits formed such as the Witswatersrand in river deposits associated with iron sulfides. This is not possible in an O2-rich atmosphere.
C. Up to 2 billion years ago the O2 produced by photosynthesis was used up by the oxidation of reduced iron, Fe2+ to Fe3+. Oxygen, an oxidizing agent, is an electron acceptor. Iron is soluble in compounds in the Fe2+ state but insoluble in Fe3+ compounds such as Fe2O3.
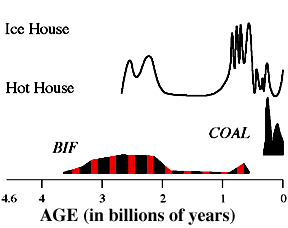
Then about 2.6 billion years ago we get the first red Banded Iron Formations (BIF's) - red and gray zones of oxidized iron layers of silica.
Responsible for world's most important iron deposits.
Cycles in photosynthesis thus produced cycles in O2 in the water column, which produced cycles in the oxidation and then deposition of Fe3+ compounds on the ocean floor. Likewise the drawdown of CO2 in the water column would produce an increase in the pH of the water and an increase in the solubility of Si. But when photosynthesis was operating at lower levels, the pH went down and the deposition of Si would take place.Formation probably due to cycles of photosynthesis then biological crash. Soluble iron comes out of solution in presence of O2 when photosynthetic microbes doing well. When they (perhaps seasonally) died off, the silica was deposited.
 |
As soluble iron started to get used up, O2 began to build up in atmosphere,
and CO2 dropped.
This continues until 2 billion years ago, when we see the first appearance of red beds. Red beds are very shallow-water, river, or soil deposits in which the iron has combined with O2 to form red iron oxide. |
These red beds indicate that O2 is building up in the atmosphere.The basic set-up of weathering as we know it was probably set up at this time.About the same time, BIFs decline, an indication that reducing compounds are disappearing from the oceans.
Experiments show that even algal scum on crushed rock enhances weathering by several times to several orders of magnitude.
VII. UNTIL ABOUT 1.9 BILLION YEARS AGO WE HAVE EVIDENCE ONLY OF THE SIMPLEST KINDS OF LIFE - THE PROKARYOTES - BACTERIA AND BLUE GREEN ALGAE
But at about 1.9 billion years we start to see fossils of much larger cells. These cells belong to the Eukaryotes - of which we are members.
Eukaryotic cells are symbiotic colonies of prokaryotes; many of the symbionts are called organelles. O2 is handled by Mitochondria, chloroplasts handle photosynthesis. These organelles independently replicate with their own genome DNA sequences in circular strands, as in bacteria. Several other kinds of organelles are similar.The relatively independent lives and separate genetic systems of these organelles led Lynn Margulis to develop the "organelle theory" (a form of which was first proposed by Mereschkowsky in 1905) in which different groups of prokaryotes became endosymbionts in other cells.
Eukaryotic metabolism can get going at about 2 % of present O2 levels,
which is consistent with the appearance of common red beds at about the
same time. Some photosynthetic eukaryotic organisms developed colonial
forms and the first "sea weeds occur". This is the first good evidence
of eukaryotic multicellularity.
The development of sex allows for species in the same sense we know them know lineages of interbreeding individuals.
About 650 million years ago (0.65 billion) the first apparently multicellular forms are present. These are called the Ediacara assemblages. They all seem to be elaborations of forms with large surface areas, all living in shallow relatively high energy environments - often in red beds.
Perhaps they needed greater surface area to absorb O2 in less-than-modern atmospheric amounts.
Although superficially similar to several animal phyla, it is unclear if ediacarans belong to extant groups. Adolph Seilacher places them all in their own phylum, which he calls the Vendizoa.The key to multicellular animals seems to the so-called homeotic genes. They are very odd and newly discovered. Seem to be involved with headness, tailness, very general properties. So head structures are homologous between flies and humans. So are eyes, even though a fly's eye is compound and ours are not, and our common ancestor had no eyes at all. The instructions for eyeness is evidently homologous. This sophistication is probably why multicellularity took so long.
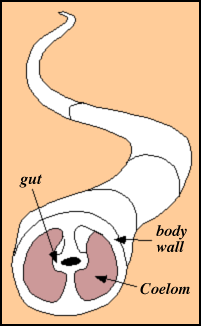
About the same time (650 million years ago) the first burrows appear.
This records the evolution of the coelom: a sac that contains the organs. We have one. It allows for hydrostatic support of the body so it can push through mud, etc.E. In the Proterozoic, the Earth's climate seems to have gone through great swings. There may have been glaciers in the tropics at times, alternating with times of more normal carbonate deposition as we see today in the warm regions. This suggests oscillating CO2 levels. I suspect that CO2 levels were basically at the whim of tectonic events during this period: undamped forcing with few damping negative feedbacks resulting in dramatic oscillations. This is a major focus of research.
This allows larger organisms that can have a hydrostatic skeleton and the ability to bend and twist and push even though they are large. Major modification of the sediments result. And oxidative processes can now go on at depth. Hence the efficiency of use of carbon fixed by photosynthesis increases.The basic structure of a coelom.
Now animals and plants could modify the physical structure of their environment, not just its chemistry - and eat each other.
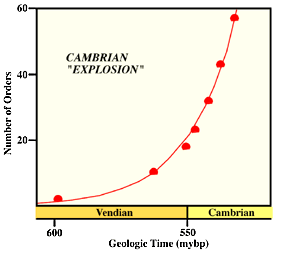 |
VIII. At the beginning of the Cambrian 540 million years ago multicellular
animal life explodes!
There is a massive increase in the kinds of multicellular animal present. Could be due to the first wave of multicellular plants on land - perhaps lichens - but very poor evidence. But that would increase the burial rate of organic carbon which would allow for many more animals in the oceans. |
This is the Cambrian explosion. A famous example is the Burgess Shale from British Columbia. It looks like most major animal phyla, perhaps all of them, were around by the middle Cambrian. By the end of the Cambrian the Phanerozoic pattern of marine organisms was pretty much established.Evolution of taxa is very rapid - looks exponential.
IX. EVOLUTION OF TAXONOMIC DIVERSITY DURING THE REST OF THE
PALEOZOIC HAS A VERY INTERESTING PATTERN.
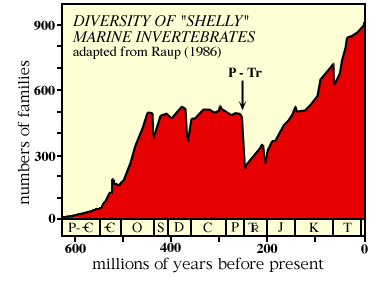
The above figure shows diversity of families of shelly marine animals during the Phanerozoic. P - Tr marks the end-Paleozoic (=end-Permian) mass extinction.
Basically, it looks like after an explosive start, the diversity of life pretty much leveled out, with a few downward blips (actually mass extinctions).